CSE 2009: Coupled Modeling of the Left Ventricle and the Systemic Circulatory System
June 15, 2009
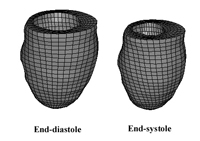
Figure 1. The left ventricle finite element model in the end-diastolic (left) and end-systolic (right) configurations.
Alexander I. Veress, Gary M. Raymond, Grant T. Gullberg, and James B. Bassingthwaighte
Finite element-based computational models of the heart have led to a greater understanding of regional deformation and function in both the normal and the diseased heart. These highly developed models incorporate patient-specific geometry as well as the fiber-sheet microstructure of the myocardium and are commonly used to study alterations in myocardial contraction resulting from cardiac disease processes. The primary limitation of FE models of the heart is that they are not connected to circulatory system models; as a result, the pressure boundary conditions are applied a priori and so are not able to simulate and respond to changes in the cardiovascular system.
To circumvent this problem, Kerckhoffs et al. [2] created a coupled system in which a 1-D circulatory system model is coupled to a 3-D finite element-based right and left ventricle (LV) model. They used the Continuity simulation system (http://www.continuity.ucsd.edu/Continuity), which represents a complete solution for the coupling of an FE heart model to a circulation model within a single simulation system.
The focus of our presentation at this year's SIAM Conference on Computational Science and Engineering was to demonstrate a similar coupling of a 1-D lumped-parameter system to a 3-D finite element-based model, but without the need to have the two systems within the same simulation package. Such a coupled system has to be time-varying, and it needs to mimic the responses of a 3-D circulatory system to the contraction of a 3-D ventricle. The work presented and briefly described here can be seen as a first step toward a system of this type; it demonstrates the linking of a circulatory model run under the JSim analysis package (www.physiome.org) to an LV model developed for and analyzed in the nonlinear, large-deformation finite element package NIKE3D [3].
The FE LV model (Figure 1) was based on the geometry of a normal 25-year-old male (high-resolution CT). Realistic material models, parameters, and fiber definitions were used to represent the myocardium [5]. An active contraction model formulated by Guccione et al. [1,5] provided the active contractile stress responsible for systolic contraction in the model.
The JSim model of the human systemic circulation includes a left ventricle and left aorta of varying elastance and a systemic arterial, capillary, and venous system in a closed loop [4]. Elastance is defined as a time-varying stiffness parameter in the relationship of LV pressure to LV volume. The parameters of the circulatory model were adjusted to provide a passive LV pressure�volume curve that at end diastole was the same as that of the FE model. The elastance parameters of the JSim model were adjusted to provide a set of normal systolic pressure�flow functions at the aortic valve. At four time points during systole, the steady-state time-varying solutions to the JSim model were used to obtain the values for the LV volume and pressure. These values were stored in an interface or information-transfer program between JSim's 1-D circulatory model and NIKE3D. The interface program supplied a 3 by 4 array (4 times, pressures, and volumes) to the FE model program. At each of the four time points, the volume output of the FE model was optimized by adjusting the active contractile stress until pressures and volumes matched those provided by JSim's circulatory model. This resulted in a dual success: the development and parameterization of an FE model with constrained time-dependent physiological outflow impedance, and a 1-D equivalent model running under JSim defining systemic arterial pressures and flows. (See Figures 2 and 3.)
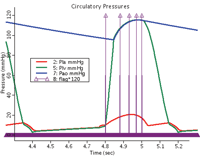
Figure 2. JSim-derived LV pressure curve (green) used in the optimization. The aortic pressure curves (blue) and the left atrial pressure curve were defined in the JSim circulatory system (red).

Figure 3. (A) JSim-derived LV volume curve (green) used in the optimization. The vertical arrows indicate the diastolic and four systolic time points used in the optimization. (B) Matching of the JSim systolic volume values (green) was achieved after four iterations for all of the time points using a 1.5-ml tolerance.
Currently, such multiscale modeling occupies only a research niche, but we foresee a time when closed-loop systems like that described here will provide useful information about normal and altered cardiovascular function. Furthermore, such work represents an important step toward the patient-specific models that would be required for clinical application. True subject-specific modeling, however, will continue to require clinical imaging (tagged MRI) of the patient, with an FE model of the patient's heart that reproduces the regional variations in deformation evident in the native heart. This can be accomplished through optimization of the material parameters of the material model in such a way that the model matches the deformation information obtained from the imaging study. This has been successfully demonstrated by Walker et al. [6,7], who studied the effects of aneurysm plication as a repair for dilated myocardial infarction in a sheep model. They utilized a two-step optimization procedure in which the differences in the end-diastolic and end-systolic volumes measured using the MRI images and those predicted by the FE models are minimized to within a set tolerance through modifications of the material parameters. They fine-tune the material parameters by minimizing the circumferential strain RMS errors calculated from the predicted FE model strains and those determined by the tagged FE analysis.
References
[1] J.M. Guccione and A.D. McCulloch, Mechanics of active contraction in cardiac muscle: Part II---constitutive relations for fiber stress that describe deactivation, J. Biomech. Eng., 115 (1993), 82�90.
[2] R.C. Kerckhoffs, M.L. Neal, et al., Coupling of a 3D finite element model of cardiac ventricular mechanics to lumped systems models of the systemic and pulmonic circulation, Ann. Biomed. Eng. 35:1 (2007), 1�18.
[3] B.N. Maker, R.M. Ferencz, and J.O. Hallquist, NIKE3D: A nonlinear, implicit, three-dimensional finite element code for solid and structural mechanics, Lawrence Livermore National Laboratory Tech. Rep. UCRL�MA, #105268, 1990.
[4] M.L. Neal and J.B. Bassingthwaighte, Subject-specific model estimation of cardiac output and blood volume during hemorrhage, Cardiovasc. Eng., 7:3 (2007), 97�120.
[5] A.I. Veress, W.P. Segars, J.A. Weiss, et al., Normal and pathological NCAT image and phantom data based on physiologically realistic left ventricle finite-element models, IEEE Trans. Med. Imaging, 25:12 (2006), 1604�16.
[6] J.C. Walker, M.B. Ratcliffe, P. Zhang, et al., MRI-based finite-element analysis of left ventricular aneurysm, Am. J. Physiol. Heart Circ. Physiol., 289:2 (2005), H692�700.
[7] J.C. Walker, M.B. Ratcliffe, P. Zhang, et al., Magnetic resonance imaging-based finite element stress analysis after linear repair of left ventricular aneurysm, J. Thorac. Cardiovasc. Surg., 135:5 1102 e1�2 (2008), 1094�1102.
Alexander Veress is a research assistant professor in the Department of Mechanical Engineering and an adjunct faculty member in the Department of Bioengineering at the University of Washington. Gary Raymond is a senior computer specialist in the Department of Bioengineering at the University of Washington. Grant Gullberg is a senior staff scientist at Lawrence Berkeley National Laboratory and an adjunct professor of radiology at the University of California, San Francisco. James Bassingthwaighte is a professor of bioengineering at the University of Washington and director of the National Simulation Resource and its JSim development.

