Mathematics Meets Oncology and Immunology
October 18, 2009
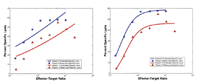
Model validation with human data. Power-law (left) and rational (de Pillis�Rad) law (right) predictions of tumor cell death (lysis) at different immune cell/cancer cell population levels. In each graph, two simulations (smooth curves) are plotted, along with data for two patients receiving treatment (squares for�one patient, triangles for�the other).�Comparison of the graphs shows a much better fit to the experimental data for the rational law than for the�power law. From �A Validated Mathematical Model of Cell-Mediated Immune Response to Tumor Growth,� Lisette de Pillis, Ami Radunskaya, and Charles Wiseman, Cancer Research, Volume 65, Number 17, 2005; courtesy of Lisette de Pillis.
Michelle Sipics
As a mathematics professor at the undergraduate-only Harvey Mudd College, Lisette de Pillis has two overarching professional concerns: advancing her research, and providing a solid education in both theory and research to her students. In an invited talk at the 2009 SIAM Annual Meeting, "Modeling Cancer�Immunology Dynamics," de Pillis described the evolution of her work in cancer research---including early projects that solidly addressed her first concern and more recent efforts that have unquestionably benefitted both. (See the sidebar below for a look at de Pillis's role in a new student program at HMC.)
De Pillis, whose earlier work was in computational fluid dynamics and parallel processing, got interested in cancer immunology about ten years ago. Charles Wiseman, an oncologist and immunologist then at St. Vincent's Cancer Treatment Center in Los Angeles, had convened a group of physicians to learn about mathematics and cancer immunology; one of the physicians contacted mathematician Ami Radunskaya for help in interpreting papers on the subject. Radunskaya, currently a professor of mathematics at Pomona College who goes by "Dr. Rad," in turn contacted de Pillis.
For de Pillis and Radunskaya, Wiseman was a rarity: a practicing doctor with an interest in using mathematics to help cancer patients. The two mathematicians initially spent their time studying the current literature and explaining the research for the physicians before realizing that they had their own contributions to make.
Tumor Dynamics
One of the first problems de Pillis and Radunskaya examined for Wiseman and his group was the asynchronous response of tumors to chemotherapy: Why do some tumors grow during treatment and others shrink after the treatment stops?
The two began by developing a three-population model that included interaction between immune cells, tumor cells, and normal cells, and took into account the competition for resources between tumor and normal cells. Previous models had considered only two populations, without competition for resources.
Analyzing their model to find null surfaces for each cell type, they found that there were multiple equilibrium points. Of particular interest was a region with one unstable and two stable equilibrium points: a time during treatment at which the patient's tumor could be driven either to steadily shrink or to grow. As de Pillis explains, such regions---where two basins of attraction exist---are of major interest to physicians.
At this point, de Pillis and Radunskaya added treatment, in the form of chemotherapy, to their model. The model now included four populations: immune cells, tumor cells, normal cells, and chemotherapy drugs, which negatively affect all the other populations. The resulting model, in which explicit delays are not included, captures the asynchronous response of tumors to chemotherapy.
De Pillis and Radunskaya then moved on to the next logical question: Now that they had a model including not only external input (i.e., chemotherapy or radiation) but also the innate contributions of the immune system in fighting the disease, could they devise a better treatment schedule?
In Denver, introducing an optimal control approach to the problem, de Pillis gave the example of two patients with similar tumors receiving the same treatment of pulsed chemotherapy---the form commonly used in cancer treatment---in which the medication is given at spaced intervals. One of the patients had begun treatment with a stronger immune system; her tumor continued to shrink after the treatment was stopped. In the patient whose immune system was initially weaker, however, the tumor re-grew.
An optimal control approach might be especially beneficial for the patient with the weaker immune system: With the same amount of medicine administered on a different schedule---one designed to maintain the desired levels of various cell populations, for example---the patient's tumor could be pushed to shrink, de Pillis says. She compares the model of immune and tumor cells to the "foxes and bunnies" predator�prey models typically used in dynamical systems.
"The underlying ideas are very similar with these cancer models," she says. "One way to look at solutions is through time---over time, how do these grow or shrink? But another way is to look at the two populations in proportion to each other, with time taken out of the equation.
"The more foxes you get, the less bunnies you have until the foxes eat too many bunnies and start to starve. You see circular patterns. And in these models, you can also have points where the populations are just in complete equilibrium: the right balance of foxes and bunnies, or in this case, immune cells and tumor cells."
By adjusting the initial parameters of the models, de Pillis explains, researchers can find those points of stability, which could lead to personalized treatment schedules. A patient-tailored chemotherapy schedule, for example, could be based on the state of the patient's immune system at the onset of treatment---on lymphocyte counts, say, or on other indicators of immune system strength. Currently, patients are simply treated according to body mass; but with this new method, doctors could be much more precise in determining the optimal treatment approach. Noting in her talk that the medical community is particularly interested in this research, de Pillis issued a "plea to the math community" to consider working on the problem.
Improved Immunotherapy
De Pillis has also collaborated with Radunskaya to study the dynamics of T-cells---immune cells that specifically attack cancer. Unlike natural killer (NK) cells, which attack any cells dissimilar to themselves, T-cells recognize and target tumor cells specifically. While NK cells are always present in the body and are activated by tumors, the presence of tumor cells is required for T-cells to proliferate.
Previously, researchers worked under the assumption that the dynamic between T-cells and tumor cells was essentially a power law: that the actual number of immune cells and tumor cells was what mattered. De Pillis and Radunskaya, with their "de Pillis�Rad Law," demonstrated that the dynamic between the two populations is in fact a rational kill law, where the relative sizes of the two populations are key.
"The effectiveness with which the immune cells can kill the tumor cells really depends more on the ratio [of cells] than just the total number," says de Pillis. "We found that as we tried to fit to the data that the ratio dependence was important.
"The other piece that's important is that there's a saturation effect," she continues. "You can have a certain number of immune cells going after the tumor cells, but eventually when you get to a certain number of immune cells, they can only kill so many [tumor cells]."
They validated their model with both mouse and human data, achieving a fit far superior to the power-law prediction. This model could also be used to improve cancer treatment, de Pillis says: Doctors could design treatments to maintain the optimal ratio of T-cells to tumor cells, as opposed to setting goals based on the actual cell count.
De Pillis also highlighted roles that immunotherapy and vaccine therapy could play in cancer treatments. Although many of her previous models treated the entire immune system as one collective population, she explained, more nuanced ways of studying the immune system's contributions and components continue to emerge. (She also noted that even a decade ago, the medical community did not put much stock in the idea that the immune system had much to do with cancer. Now, however, researchers recognize the important role played by T-cells in fighting the disease.)
De Pillis is also currently involved in efforts to model B-CLL: B-cell chronic lymphocytic leukemia, a cancer of the immune system characterized by the accumulation of large numbers of white blood cells---B-cells---although the cells' high proliferation rate is more serious than the number of them. The precise cause of the disease is unknown, and there is currently no cure.
It might seem counterintuitive to consider immunotherapy for B-CLL. As de Pillis puts it, "If you have a disease of the immune system, how do you then counter it with immunotherapy?" In fact, though, she's working on modeling the dynamic between B-CLL and specific immune cells, which could lead to new treatment models.
"The immune system has multiple components to it," de Pillis explains. "Current research is looking into treating [B-CLL] with both natural killer cell treatment and T-cell treatment. We're developing the foundational model to see if we can get a validated model of B-CLL interaction with these two components of the immune system, and then see if we can model treatment."
She's also part of a separate study of dendritic cell therapy. Dendritic cells can prime T-cells to attack a patient's specific tumor cells---serving, in theory, as cancer vaccines. Although very promising in the lab, this form of immunotherapy doesn't perform as well in a clinical setting. De Pillis hopes that mathematics will help uncover the reasons for the discrepancy between lab and clinical results so that researchers can devise improved treatment regimens.
Bridge-building Mathematicians
As much as she enjoys the mathematics itself, de Pillis says it's that potential applicability---whether in determining a better chemotherapy schedule or developing a cancer vaccine---that's a major factor in her decision to work on these particular projects.
"I think certainly mathematics by itself is of value. Just doing mathematics for its beauty is of value; very often the practical application shows up maybe a century later," she says. "But a lot of what motivates me is the idea that this could actually be helping people. We might be discovering something that will make life better for some people suffering some disease."
For mathematics to continue to contribute on a large scale, de Pillis stresses the importance of interdisciplinary efforts and reaching out to experts in other fields.
"We need to build those bridges between the mathematical community and the medical community," she says. "We've gone to medical conferences and been very surprised at how well received our work has been---but often we've been the only mathematicians at these conferences.
"I think mathematics has a lot to offer, but the way we'll be able to make our work really useful is to make it accessible to the folks who are working in the clinic. It has to be accessible and understandable--we need to learn to speak their language."
Michelle Sipics is a contributing editor at SIAM News.
Trailblazing Math Clinic Offers Research/Career Opportunities
The long-running Harvey Mudd College Mathematics Clinic has been recognized by the American Mathematical Society as a "trailblazer" for its efforts to introduce math majors to real-world problems and for giving students "a terrific research experience as well as a glimpse at possible future careers." The math clinic is an extension of the even longer running HMC Clinic Program, in which teams of advanced engineering students develop solutions to open problems presented by sponsoring organizations. Working with a faculty adviser and a liaison from the sponsor over the course of an academic year, a team of clinic students collectively devotes between 1200 and 1500 hours to the program.
Lisette de Pillis knows firsthand how useful the clinic experience can be for students: She was a faculty adviser to a team of students who worked on spatial tumor modeling in a project for Los Alamos National Lab, where physicist Yi Jiang was also developing cancer models. (For more about Jiang's work, see "Step by Step, Math Models Unlock Secrets of Cancer Biology," SIAM News, October 2006.)
"The [students' task] was to [model] vascularization and its effects, and they tried different kinds of things," says de Pillis, explaining the many factors to be considered: What changes with the size of the tumor? How do you do this modeling in three dimensions? And because blood vessels transport both nutrients and chemotherapy medication, how does vascularization---the growth of blood vessels---affect the transport of the drugs?
"I was amazed to see how creative and successful these students could be in addressing these open research questions," de Pillis says.
The clinic program that supported that research has expanded once again: In 2005, HMC inaugurated the Global Clinic program, giving HMC students from all departments the chance to collaborate with student teams from institutions around the world. Earlier this year, de Pillis was named director of the Global Clinic.
The standard clinic program runs during the school year, from September to May. The global program begins in June, when HMC students spend three weeks at a partner institution, working on project planning, communication, and team-building activities. The partner students return the visit the following month to continue project planning and present a design review to the sponsoring organization before the academic year begins.
Amgen and Hewlett Packard are among the organizations that sponsored pilot projects during the Global Clinic's first two years; in both cases, participating HMC students teamed up with students at the University of Puerto Rico Mayag�ez. In a project for Applied Biosystems, another early sponsor, HMC students worked with students from the National University of Singapore. In 2009�10, groups of students will again work with teams in Singapore, this time on wastewater treatment in China and sensor placement near volcanoes, as well as with students in Iceland, on topics in renewable energy.
De Pillis is hopeful that the global program will expand on the experience the initial clinic program has provided for more than four decades.
"Students have to learn how to communicate, write reports, structure their team, manage timelines," she says. "They work with a faculty adviser who keeps them on track, but [they] do the bulk of the underlying work. These kids really come through."
For more information on the Global Clinic program, readers can visit http://www.hmc.edu/academicsclinicresearch/clinicprogram1/globalclinicprogram.html
---MS.

