Materials Science: New Materials for Energy Systems: Mathematical Challenges
September 15, 2010
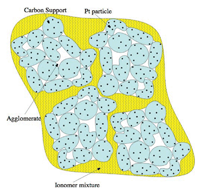
Figure 1. Schematic diagram of the catalyst layer of a PEM fuel cell.
Hydrogen fuel cells could play an important role in the so-called new (post-fossil fuel) energy economy. Power generated from renewable sources (solar, wind, tidal, biomass) or nuclear stations would be converted into hydrogen for storage. The hydrogen would then be used in fuel cells to generate electrical power for stationary, automotive, and portable power applications.
Fuel cells are an established technology. Before they can see widespread usage, however, cost reduction, increased durability, and hydrogen infrastructure (large-scale production, efficient storage, and a distribution network) need to be resolved.
The electrochemical reaction (on a platinum or platinum alloy catalyst) of hydrogen with oxygen from air can occur at relatively low temperatures. Fuel cells differ from batteries in that reactants are fed into them and they can be run continuously. The only product of the reaction is water, which makes these devices environmentally friendly.
Fuel cells and many other electrochemical systems (including batteries) are inherently multiscale. Small (micro- or nanoscale) structures are present in electrodes to increase the surface area available for the electrochemical reactions and to allow the efficient, multicomponent (and possibly also multiphase) transport of reactants to and products away from these sites. An example of such a structure, a cathode catalyst layer in a polymer electrolyte membrane (PEM) fuel cell, is sketched in Figure 1.
The layer is formed with functionalized polymer (ionomer) that can transport protons from the anode (the hydrogen side). Also present are carbon particles (sub-micron-scale) in which platinum particles (nanoscale) have been embedded. Protons from the anode move through the ionomer (which conducts protons but not electrons); electrons move through the carbon particle network, which is connected through the external circuit; and oxygen gas moves through gas pores (not shown in the figure). All these elements must meet at platinum catalyst sites for the reaction to occur. Product water is removed through the pores as vapor or liquid.
Water is both necessary and problematic in these devices: For good proton conductivity, the ionomer must be well hydrated, but too much liquid water clogs pores or gas channels. "Water management" is a key issue for PEM fuel cells; understanding it requires consideration of mass and heat transport at the device level.
The development and improvement of structures like the PEM catalyst layer have proceeded almost entirely by experimental trial and error. Very few modeling efforts have included any details of the small-scale structures. Some of the cutting-edge modeling work that is under way was presented at the meeting: Karen Chan, who works in Michael Eikerling's group at Simon Fraser University, discussed nanoscale reaction behavior near platinum particles in PEM fuel cells; Hsun-Yi Chen, of Katsuyo Thornton's group at the University of Michigan, discussed catalyst agglomeration in solid oxide (high-temperature) fuel cells. Maarten Biesheuvel of Wageningen, the Netherlands, discussed the nanoscale performance of an electrochemical technique for water desalination; Keith Promislow and Nir Gavesh of Michigan State presented novel theoretical models of the formation of proton-conducting pores in functionalized polymers that are present in fuel cells, solar cells, and many other devices. The speakers made it clear that all this work on different applications has a common, mathematical framework.
The materials discussed so far in this article are components of larger devices. The cathode catalyst layer can have a thickness of the order of 25 microns and cover an area as large as 1000 square centimeters in a unit fuel cell (see Figure 2).
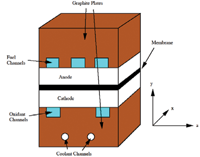
Figure 2. A unit fuel cell (the aspect ratio is not to scale).
Up to hundreds of unit cells can be stacked in electrical series to make a fuel cell stack for use in stationary and automotive power. A side view of such a stack is shown in Figure 3.
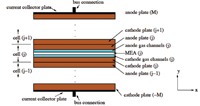
Figure 3. A PEM fuel stack.
In my plenary talk, I outlined the success of a group project (with Ballard Power Systems, supported by additional funding from the Canadian industrial mathematics network MITACS) to develop computational models of PEM fuel cell stacks. Careful experiments were done to characterize the local behavior of catalyst layers and other components with complex microstructure. These components were then represented in the stack-level model by empirical functions fitted to the experiments. The conditions experienced by these components at different places in the stack are determined through coupling of the local behavior with stack-level mass transport (laminar channel flow), electrical effects (currents in the plates between cells), and thermal transport (to coolant channels between cells). Effects of reactant gas flow sharing from common headers are also included. The resulting model, which includes many of the important effects in PEM fuel cells, was validated against independent experiments. At the meeting Jay Benziger and Ioannis Kevrekidis of Princeton University discussed their detailed experimental work on PEM fuel cells, which has also led to predictive component models.
The sessions on energy systems at the meeting served to introduce questions---concerning both theory and applications---of interest to the wider materials science audience. These and other talks were also a reminder of the amounts of time and money spent in the experimental development of new materials. Any theoretical insights into ways to improve these materials (how to construct cathode catalyst layers for PEM fuel cells, for example) would be a major contribution. If the mathematical materials science community is to receive the recognition it deserves from the wider scientific community, some concrete success of this type is needed.
This year's SIAM conference on materials science, the sixth in a well-established series, was the first official activity of the new SIAM Activity Group on Mathematical Aspects of Materials Science. Articles about the SIAG and some of the conference activities appeared in the July/August issue of SIAM News; additional articles will follow later this fall.

