ICIAM 2011: Our �Second Brain�: Modelling its Development and Disease
December 13, 2011
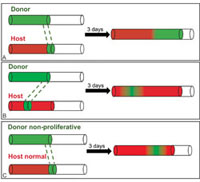
Figure 1. Schematic profiles of chick�quail graft experiments and two modelling approaches. Host neural crest cells, or NCCs, are shown in red, donor NCCs in green. Donor NCCs are grafted into the wavefront (A) and behind the wavefront (B). In (A) and (B), NCCs at the wavefront are responsible for driving further colonization, called frontal expansion. (C) Donor NCCs with no proliferative ability are grafted into the wavefront. Host NCCs overtake the non-proliferative donor cells and subsequently have proliferative opportunity to drive further colonization. (In variants tested experimentally, the donor tissue is removed from behind instead of at the wavefront. The same results hold for these variants.)
The enteric nervous system (ENS), nicknamed the "second brain" [2], is a large, complex network of neurons lining the wall of the vertebrate gastrointestinal tract, where it controls normal gut function and peristaltic contraction.
During embryonic ENS development in vertebrates, a small population of neural crest cells (NCCs) migrate from the hindbrain to colonize the gut wall from the stomach to the anal end. Hirschsprung's disease (HD), a relatively common birth defect (1/5000 births), occurs when NCCs fail to colonize to the anal end. This causes intractable constipation and is potentially fatal unless surgically treated.
During the highly time-tabled colonization process (approximately 4 weeks in humans, starting at week 3; 4�5 days in rodents and avians), there is a vast increase in NCC numbers through cell division (proliferation), up to a preferred density. At the same time, the mesenchymal cells making up the gut tissue undergo cell division, resulting in a massive elongation of the intestine. Although the colonization wave is strictly time-tabled and predictable, individual NCC movement is unpredictable in speed and direction.
Don Newgreen, who heads an embryology laboratory at Murdoch Childrens Research Institute (associated with the Royal Childrens Hospital), approached us, thinking that mathematical modelling might lead to better understanding of the NCC colonization process and the cause of HD. Over several years, he and I, together with our research groups, have addressed various aspects of ENS development, two of which are discussed here.
For this collaboration we have developed both macroscopic population-level models (partial differential equation models) and microscopic cellular-level stochastic models (cellular automaton/agent-based models). In the PDE models, the cell density changes in time due to a flux term (e.g., linear diffusion resulting from NCC random motion), a logistic growth term (from cell proliferation up to a preferred density), and an advective term (from uniform domain growth with gut elongation). In the cellular automaton models, probabilities are assigned to cell proliferation, with at most one NCC per lattice site (i.e., an exclusion process). We developed a discrete growing domain model, in which the underlying lattice sites represent the gut tissue cells. To model gut elongation, we grow the lattice [1] by randomly inserting new sites into each row of the lattice.
Successful or Failed Colonization?
In the absence of gut growth, we can determine how NCCs behave in different parts of the colonization wave using quail and chick NCCs, which are functionally equivalent but respond (and therefore label) differently to various antibodies. We suggested experiments to test against our models.
Without gut growth. In tissue culture in which there is no gut growth, a segment of chick host gut tissue is removed and replaced with quail donor tissue containing NCCs. Three days later the resulting host and donor cells are identified. Donor cells can be placed at or behind the wavefront (Figure 1A,B). The models and experiments give the same results. Cells at the wavefront are responsible for an increase in cell numbers in unoccupied regions, thereby driving the colonization wave; we call this "frontal expansion." We conclude that proliferation at the wavefront is the driver of colonization [5].
What happens if NCC proliferation is eliminated at the wavefront (as shown in Figure 1C)? Donor cells can be treated to eliminate cell division, but with cell motility preserved. The experimentalists envisioned two possible outcomes: Either nothing would happen (stall) or the host cells would proliferate and push the donor cells forward (shunting). In contrast, our models predict that the donor cells will diffuse, leave space for the host cells to move into and eventually overtake the donor cells, proliferate at the wavefront, and drive further colonization. The experimentalists were surprised at their results, which matched the model predictions [5].
With gut growth. The models predict that colonization will again proceed by frontal expansion; now, however, proliferation also occurs behind the wavefront because of the additional space produced by domain growth. Successful colonization occurs only if the NCC proliferation rate is sufficiently large compared with the domain growth rate. The predictions were confirmed experimentally [6].
These results have important implications for HD. We now understand HD to be a defect of NCC proliferation and not, as thought previously, a defect of NCC motility. Furthermore, we have identified proliferation as the common link between the known HD genes. In particular, stochastic effects can determine the success or failure of the colonization process for a certain range of NCC proliferation rates. This may explain the existence of pairs of identical twins in which only one has HD. Finally, stem cell treatment of HD, as an alternative to surgery, is more tractable if NCC proliferation is the key mechanism.
Mesoscale Structure of the NCC Colonization Wave
Images show that NCCs are in close contact and form chains that intersect, establishing a network that is spatially stable over many hours. However, the NCCs making up the chains move independently and change neighbors unpredictably on the scale of minutes. Recent experimental observations imply that NCCs have an affinity for nerve fibres (axons). After eliminating the possibility that NCCs follow some underlying chain-like network, we proposed two alternative hypotheses: (i) axons form the paths and NCCs assemble along the axons, or (ii) NCCs form chains and axons follow NCC chains. We discuss (i) here.
Additional biological features must be included in the models. NCCs progressively convert to neurons, and each neuron extends an axon. Axon elongation occurs by growth at the tip, called the "growth-cone." The gut tissue produces a growth factor required for NCC function. We propose that NCCs deplete the growth factor, thus producing a growth-factor gradient across the wavefront. Because axon growth-cones are known to respond to growth-factor gradients, this mechanism gives a directional forward bias to axon extension.
When these features are translated into cellular automaton rules, a stable chain-like network of NCCs is generated and the key characteristics (Figure 2) at both the population and cellular levels are reproduced [4].
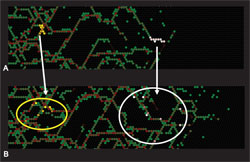
Figure 2. (A) Starting from just a few NCCs, axons (red lines) form a connected network; NCCs (green) largely occupy sites along the axons, so that the NCCs form an interweaving chain-like network. The axons enclose open, often empty spaces. This network pattern is stable over time. (B) Two groups of NCCs are labelled (white and yellow) at this time. At a later time, the NCC and axon network continues to evolve. The labelled NCCs become separated, but remain on the stable evolving network. Frontal expansion is maintained, but now occurs on a mesoscale structure.
The enteric nervous system is a fascinating developmental system that offers many interesting modelling challenges. Our two modelling approaches have raised new theoretical questions related to both interacting particle systems and nonlinear diffusion equations. Currently, our collaboration is focused on later stages of ENS development [3].
References
[1] B.J. Binder, K.A. Landman, M.J. Simpson, M. Mariani, and D.F. Newgreen, Modeling proliferative tissue growth: A general approach and an avian case study, Phys. Rev. E, 78 (2008), 031912.
[2] M.D. Gershon, The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine, HarperCollins, New York, 1998.
[3] E.J. Hackett-Jones, K.A. Landman, D.F. Newgreen, and D. Zhang, On the role of differential adhesion in gangliogenesis in the enteric nervous system, J. Theor. Biol., doi:10.1016/j.jtbi.2011.07.013 (2011), in press.
[4] K.A. Landman, A.E. Fernando, D. Zhang, and D.F. Newgreen, Building stable chains with motile agents: Insights into the morphology of enteric neural crest cell migration, J. Theor. Biol., 276 (2011), 250�268.
[5] M.J. Simpson, K.A. Landman, B.D. Hughes, and D.F. Newgreen, Looking inside an invasion wave of cells using continuum models: Proliferation is the key, J. Theor. Biol., 243 (2006), 343�360.
[6] D. Zhang, I.M. Brinas, B.J. Binder, K.A. Landman, and D.F. Newgreen, Neural crest regionalisation for enteric nervous system formation: Implications for Hirschsprung's disease and stem cell therapy, Dev. Biol., 339 (2010), 280�294.
Kerry Landman is a professor in the Department of Mathematics and Statistics at the University of Melbourne, in Victoria, Australia.

