Mathematical Modelers Help Plumb Mysteries of Parkinson's Disease
May 30, 2003
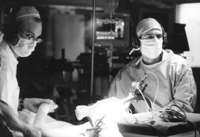
Deep brain stimulation. Performing DBS surgery, Erwin Montgomery (left) moves the patient's arm as his colleague Ali Rezai listens through headphones to the resulting neuron impulses from the basal ganglia. In this way, the doctors find the correct location for implantation of the electrode. Photograph courtesy of the Cleveland Clinic.
Dana Mackenzie
"Deep brain stimulation" might sound like a trendy kind of meditation, or perhaps the outcome of a particularly exciting mathematics seminar. But for thousands of victims of Parkinson's disease, it is a medical procedure that has given them back their lives. In this treatment, doctors implant an electrode---somewhat like a heart pacemaker---into the patient's brain.
After the operation, patients can turn on the electrode, and turn off their symptoms, with the flick of a switch. Gone are the hand-shaking tremors that kept them from getting dressed, the difficulty in moving that reduced their walks to a shuffle, the blank stare that is sometimes called "Parkinson's mask." As one patient wrote on the Web, "After 22 years, I am free of the embarrassing tremors, and if we first met, you wouldn't be able to tell I have Parkinson's. In fact, I quit announcing it at gatherings because no one notices!"
Like Parkinson's disease itself, deep brain stimulation (DBS) is a deep mystery. "We don't know how it works," said Erwin Montgomery, a neurosurgeon at the Cleveland Clinic, in an interview last year on 60 Minutes. "We really don't have the foggiest notion. We have some theories, but increasingly we're finding evidence that those theories are not true."
Recently, mathematicians have joined the effort to understand Parkinson's disease. A model developed by mathematician David Terman of Ohio State, and colleagues---including Alice Yew, Jonathan Rubin, and Charles Wilson---has explained, for the first time, where the tremors so characteristic of the disease come from, and it has also turned upside down the prevailing theory of how DBS works. Although the work is still very preliminary, Terman says, "I'd go out on a limb and say that mathematics could have an important effect on DBS very shortly---if the surgeons listen."
The Paradox of Parkinson's
So far, what doctors know about Parkinson's disease is a patchwork of experimental results and observations from fortuitous accidents. In 1961, a neurosurgeon named Irving Cooper, while operating on a patient with Parkinson's disease, accidentally tore a blood vessel and aborted the procedure. When the patient awoke, both doctor and patient were stunned to discover that his tremors had disappeared. On further investigation, Cooper found that he had destroyed a region called the globus pallidus, part of the brain's circuitry for controlling voluntary motion (collectively called the basal ganglia). For a few years afterward, removal of the globus pallidus became the treatment of choice for Parkinson's.
In the late 1960s, though, the introduction of a drug called levodopa banished surgery to a secondary role. Levodopa is converted in the brain to dopamine, a neurotransmitter not produced in sufficient amounts by Parkinson's patients. According to conventional wisdom, this deficiency allows the globus pallidus to overproduce neuroinhibitors that reduce the firing rate of motor neurons---resulting in the characteristic slowness and awkwardness of motion. Less risky than surgery, levodopa both reduces the difficulty of movement and alleviates the Parkinsonian tremors. Over the long term, however, many patients developed tolerance to the drug and their symptoms returned. "They get an on-off phenomenon, where they can walk down the street to the bus station, but then they can't get on the bus," says Karen Sigvardt, a physiologist at the University of California at Davis.
Deep brain stimulation, the newest treatment on the scene, was invented in the late 1980s by Alim Benabid, a neurosurgeon at the University Joseph Fourier in Grenoble, France. Following the usual surgical procedure for locating the globus pallidus, Benabid would use electrodes to detect the particular electric signals it emits when a patient's arms and legs are moved. But he noticed that the electrodes also seemed to quiet patients' tremors. "Doctor Benabid said, 'why should I destroy the globus pallidus, when I can control the tremor by electrical stimulation?'" Montgomery explains.
Conventional theories of the brain do not explain where tremors, probably the most noticeable and debilitating symptom of Parkinson's disease, come from, or why levodopa causes them to disappear. They fail even more conspicuously to explain why DBS, which appears to stimulate the globus pallidus (although this point is still controversial), would alleviate the symptoms. If anything, it should make them worse.
According to Terman, these paradoxes point to a fundamental flaw in the way biologists have traditionally thought about the brain's circuitry. "The previous models have been static, based on the average firing rate of the neurons," he says. In other words, they ignore the time factor. But for a disease like Parkinson's, which produces oscillations, a steady-state model could not possibly be adequate.
"What shows up in biological papers is that excitation increases firing rates, and inhibition decreases them," says Jonathan Rubin, a former postdoctoral researcher working with Terman who is now a faculty member in the mathematics department at the University of Pittsburgh. "But from a dynamical systems perspective, we think about time scales. We know that when we put together processes that happen on different time scales, we get a much richer picture."
The Dynamical Brain
According to Terman and Rubin, it is a mistake to think of chemical inhibitors merely as an "off" switch for neurons. Instead, they work more like a control knob that reduces or increases the amount of synchronization among sets of neurons. (The emergence of synchrony, incidentally, has become a major research topic for mathematicians in the past few years; see Steve Strogatz's SYNC, reviewed in the April issue of SIAM News, for examples.)
Terman's first model incorporated only two of the four types of nuclei, or clusters of neurons, found in the basal ganglia: the "external segment" of the globus pallidus (GPe) and the dime-sized subthalamic nucleus (STN). These two structures carry on a constant, unequal conversation, with the inhibitory GPe telling the STN to shut up, and the excitatory STN telling the GPe to speak louder.
Mathematicians have known for a long time that excitatory-inhibitory pairs are a recipe for oscillations. To understand why, think of a network of wolves and rabbits. The wolves "inhibit" the rabbits by killing and eating them, while the rabbits "stimulate" the wolves by feeding them. The wolves might be expected to win, but it doesn't work that way---either in nature or in mathematical models. Instead, if the wolf population gets too large, there aren't enough rabbits left to feed them, and the population crashes. With less predatory pressure on them, the rabbits then start to proliferate---creating an abundant food supply for the wolves. Then the cycle starts again.
Such boom-and-bust cycles, first noted by mathematical biologists Alfred Lotka and Vito Volterra, are common but by no means universal in excitatory-inhibitory pairs. The qualitative outcome depends very much on certain quantitative parameters: How good are the wolves at hunting? How much do wolves compete among themselves for food? How rapidly do rabbits multiply?
Thus, the first step for Terman was to get the details right. "We wanted to build as biological a model of these neurons as possible," he says. The parameters in Terman's model, which express in great detail how each type of cell responds to a particular voltage or to the flow of a particular ion, came from literature searches and experiments done by his biologist collaborators, Mark Bevan of the University of Tennessee and Charles Wilson of the University at Texas at San Antonio. (It was Wilson, in fact, who got Terman started on the project.)
In the resulting model, Terman found that two factors led to the synchronous bursting characteristic of Parkinson's tremor. One was the internal pattern of connections between GPe neurons. When the connections were more structured, the neurons would synchronize. They would either fire alternately, like the lights at a railroad crossing, or they would fire in a "traveling wave." A random architecture, on the other hand, led to a more disorganized (which in this case means normal) firing pattern.
The second synchronizing factor was the level of inhibition between the GPe neurons-analogous to the level of competition between wolves. When the interactions between GPe neurons were weakened, the whole network of GPe and STN neurons would switch from random bursting to patterned bursting. "This suggests that during Parkinson's, what happens is that the chemical changes unmask the ability of these two nuclei to generate this pathological rhythm," Terman says.
And how does DBS restore the basal ganglia's normal function? "We want to argue that it actually increases activity in the globus pallidus," Terman says. Again, though, it's not the amount of activity that matters, but the dynamics. The globus pallidus is in charge of sending information to the thalamus, the brain's relay station: "I want this muscle to contract (or relax)." Parkinson's disease corrupts the information. DBS corrupts it, too, but in a different and more benign way.
Like a ham radio operator tuning into a weak signal, the thalamus can better understand a broadcast that is full of static than a broadcast that fades in and out. The basal ganglia under Parkinson's disease is like the station that fades in and out, failing to transmit some information to the thalamus and---even worse---confusing the thalamus to the point that it mistakes the oscillations for messages. When treated with DBS, the basal ganglia is like the staticky but steady radio station that manages to get its messages through despite the interference.
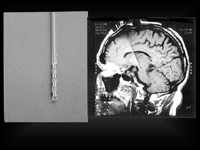
An electrode (left) and an MRI image (right) showing a brain in which an electrode has been implanted. Courtesy of Erwin Montgomery.
From Models to Human
At present, deep brain stimulation is a tremendous success story. Montgomery estimates that only 2 to 3% of his patients suffer "permanent complications" from the surgery, while about 75 to 80% show significant improvement. "And these are patients who were no longer responding to other treatments," he says. Not only that, the treatment seems to last. "The earliest patients [in the U.S.] had surgery in 1995, and they are not getting worse. That's even more impressive when you realize that Parkinson's is a progressive disease," Montgomery says.
Even so, there is room for improvement, and Montgomery has high hopes that the mathematical models will help. There is still some debate about the best site for the electrodes, the globus pallidus or the STN. The pattern of stimulation---the strength of the electric signals, the shape and duration of the wave forms---is determined patient by patient, and mostly by trial and error. No one has tested yet whether more complicated or irregular patterns of stimulation might work better.
Terman is optimistic about the potential of his model, but realistic about its limitations. "This is clearly just a first-generation model," he says. "It needs to be improved as experiments come in." One high priority is to see whether his results, which have been derived and tested only for small groups of cells, scale up to the level of a living brain.
Meanwhile, at Emory University, Jerrold Vitek has been studying the effects of DBS in monkeys. Karen Sigvardt, the UC Davis physiologist, has gathered information about the brain's response to electrodes during the DBS operation, and is collaborating with mathematician Nancy Kopell of Boston University to see if the results fit mathematical predictions. Ultimately, Kopell and Sigvardt hope to understand not only the diseased state but also the normal function of the basal ganglia.
For mathematicians like Terman and Kopell, Parkinson's disease is only the first item in a much larger agenda. Rhythms, both normal and abnormal, abound in the brain. Every time we sleep, the neurons in our thalamus lock into synchronous rhythms. Abnormal rhythms occur in such diseases as epilepsy and chorea. Intuition can take biologists only so far in understanding where these oscillations come from (and how, if necessary, to stop them). After that, they will need mathematical models.
"The days when you could just look at neuronal firing patterns on an oscilloscope are long ago," says Montgomery. "We'll soon be in desperate need of mathematicians who understand biology."
Dana Mackenzie writes from Santa Cruz, California.

