Cell-level Math Modeling Fuels Progress in Biomechanics
March 2, 2002
Sara Robinson
It's a strange world, one in which tiny creatures use extraordinary measures to propel themselves through an extremely viscous environment.
Some of the creatures live in large colonies and move by grabbing onto their neighbors and pulling themselves forward. Still others drill their way through the goop, using projecting filaments, shaped like stretched-out corkscrews, to propel themselves.
Such is the world of molecules and single-celled organisms, such as proteins and bacteria. The study of the tiny "motors" of this world, known as cellular and molecular biomechanics, is the specialty of George Oster, a professor of molecular and cellular biology at the University of California, Berkeley. Although Oster's academic appointment is in a biology department, his background is in physics and mechanical engineering.
Working among biologists, Oster says, enables him to be familiar enough with the state of biological research to develop mathematical models that take all the known data into account. In his papers, which appear primarily in biology journals, he explains his work largely in words and pictures, relegating the equations to the appendix.
Life at Low Reynolds Numbers
Biomechanics is a far cry from the world of automobiles and jet planes. Indeed, the physical principles governing the motion of single-celled organisms seem bizarre and counterintuitive to large creatures like us.
For tiny, lightweight beings, viscous forces, the tangential forces exerted by the fluid in the surrounding environment, completely dominate inertial forces, which are proportional to mass. Worlds dominated by viscous forces are often referred to by the small value of their Reynolds number, the unitless ratio of inertial forces to viscous forces in a fluid.
"Life at low Reynolds number is counterintuitive," Oster says. "It's like being in a swimming pool full of thick honey, swimming at a rate of one stroke a day." Oster describes it as the world envisioned by Archimedes, where forces act instantly to produce velocity, rather than the world of Newtonian physics, where forces produce accelerations.
Oster has delved into many different types of biomechanical mechanisms, from the motors that produce ATP, the source of a cell's energy, to the mechanisms by which various bacteria move. His most recent work-done with Charles Wolgemuth, his Berkeley postdoc, Egbert Hoiczyk of the Howard Hughes Medical Institute at Rockefeller University, and Dale Kaiser, a biochemist at Stanford University-offers a hypothetical mechanism for the gliding motion of two common strains of bacteria, myxobacteria and cyanobacteria. The proposed locomotion method is known as "slime propulsion," or, as the researchers sometimes call it, "the snot gun."
The group's work (not a good lunchtime read) shows that the bacteria could move at the observed speeds by exuding sticky ribbons of what the researchers call a polymer slime from nozzles located at their back ends. The slime sticks to surrounding surfaces and pushes against them, propelling the bacteria forward (see illustrations).
Understanding Bacterial Motion
Much research on bacterial locomotion stems from a desire to better understand disease: how it starts and spreads, and how to design drugs to treat it. Neither cyanobacteria nor myxobacteria, the subjects of the recent work, are pathogens. Still, understanding how these bacteria glide is important, Oster says, because many pathogens use similar mechanisms for movement. Nature, after all, has far fewer mechanisms for mobility than types of creatures.
Myxobacteria, the primary focus of the study, are also fascinating creatures in their own right, and studying their movements gives insight into their complex social behavior. "Studying movement is a valuable way of studying the behavior of bacteria or other cells," says Kaiser, who is a myxobacteria expert. "You want to know what the machinery is that gives rise to the movement. Then you can figure out how the machinery is controlled so you have a deeper understanding of the behavior."
Myxobacteria live in fertile soil, where they tend to be densely concentrated, perhaps a million bacteria in one gram of dirt from a typical backyard. They are predators. They hunt in packs, devouring other bacteria that, in turn, consume rotting plant matter.
Cyanobacteria, also an ancient strain, are not predators; they are universal primary manufacturers. Like plants, they convert sunlight, water, and carbon dioxide into sugars and proteins. Indeed, cyanobacteria were long known as blue-green algae, until further study showed that they are actually bacteria.
Biologists have defined two classes of mechanisms for gliding, "social motility" and "adventurous motility." In social motility, organisms send out arms, called pili, and attach to their neighbors, retracting the arms to generate a force that yanks them forward. In adventurous motility, organisms manage to propel themselves independently, as with slime propulsion. Myxobacteria appear to use the two mechanisms synergistically, while cyanobacteria are only adventurous.
Other researchers have proposed very different models for adventurous motility, Oster says, but only his group's slime propulsion model is consistent with all observations.
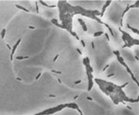
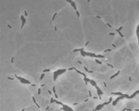
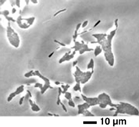
Slime trails. Deposition of slime by three strains of Myxococcus xanthus as the bacteria glide on agar. From a paper by Oster, Kaiser, and colleagues, to appear in Current Biology.
Math Model Makes Gliding Mechanism Plausible
The notion of slime propulsion was introduced in an earlier paper by Hoiczyk and Wolfgang Baumeister. They observed that cyanobacteria have nozzles that exude slime at the same rate at which the bacteria move, and hypothesized that the slime secretion might be responsible for the movement.
The paper by Oster, Kaiser, and colleagues, to appear in the journal Current Biology, builds on this observation by showing that myxobacteria also possess nozzles, more than fifty apiece, that are similar to, although smaller than, those of cyanobacteria. The nozzles are located at the front and back ends of the bacteria, appropriate positions for propulsion. Oster and Wolgemuth came up with a mechanism by which a propulsive force could be generated by the secretion of slime from the nozzles. They developed a mathematical model for this process, and showed that it generates enough force to account for the observed properties of the bacteria's motion. They also roughly estimated the metabolic cost of manufacturing slime, and showed it to be greater than the cost of propulsion.
The propulsive force is created when the slime absorbs water flowing in from outside the cell and then swells within the nozzle until it protrudes out the back. To evaluate whether the swelling of the slime could provide a strong enough force to move the bacteria at the observed rates, the researchers created a model to compute the force exerted by the swelling of the slime at the nozzle exit (the swelling pressure times the cross-sectional area of the nozzle opening). The scientists don't know the exact makeup of the slime, but they found that their results were not sensitive to the exact chemistry. The model also doesn't address how the slime gets into the nozzle, so they simply assume that the end of the nozzle is supplied with a constant fractional volume of slime.
The slime, technically speaking, is a polyelectrolyte gel. A polyelectrolyte gel is a complex substance made up of a mesh of polymers, studded with ions, immersed in a fluid solvent. Oster and Wolgemuth proposed a dynamic model that explains how a gel with such properties could react to hydration by producing a thrust.
Since the polymer mesh acts as a deformable solid, it is represented as an elastic solid, while the behavior of the solvent is governed by the Stokes equations of fluid mechanics. Also entering the equations are electrostatic forces from the ions present in the slime and in the surrounding environment.
Several forces contribute to the swelling pressure of the gel; most significant is the ion gas pressure produced when the gel hydrates. In the equilibrium state, positive charges in the fluid tend to cluster around the negative charges in the polymer. When the gel hydrates, however, the positive charges diffuse outward, and the negative charges, still attached to the mesh, try to follow them. This force stretches the polymer taut. Also swelling the gel is the entropic force resulting from the tendency of the gel fibers to mix with the surrounding fluid. Resisting the swelling are the elastic forces of the fiber mesh and attractions between the gel fibers.
After computing the swelling pressure, the authors estimated the drag force that must be overcome by the propulsive thrust, assuming the bacterium to be a cylindrical filament in a fluid of constant high viscosity. They showed that the propulsive force is strong enough to overcome the drag and propel the bacteria forward at the observed rates.
Finally, they produced a rough estimate of the metabolic cost of the mechanism, calculating the rate at which slime monomers come out of each nozzle and multiplying it by the bond energy between the monomers when they are polymerized. The result is far greater than the energy cost of the propulsion.
This estimate, though, is the weak point in the paper, Kaiser says. To "prove" the mechanism, the researchers will have to figure out the process by which the slime is made. His group at Stanford is now using a process called "random mutagenesis," a random knocking out of genes, to see how the deletions affect the ability of the mutant bacteria to glide. In this way, they hope to isolate the genes (50-100 by his estimate) responsible for the mechanism and learn what proteins they make.
If biologists can achieve a better understanding of the biological aspects of the slime production process, then a more precise model of the mechanism can be developed. The mathematical model plays a key role because it "makes the slime propulsion mechanism plausible," says Kaiser. "Initially, it doesn't sound like a great engine, but the way George Oster has figured it out, you really see how slime propulsion could push a bacterium along."
Sara Robinson is a freelance writer based in Berkeley, California.

