A Gem of a Definition
January 8, 1998
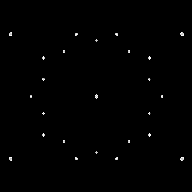
Figure 1. Simulated diffraction of the "quasicrystal" that is the vertex set of a Penrose tiling.
Barry A. Cipra
Marjorie Senechal is an expert on the mathematics of crystalline structures. A professor of mathematics at Smith College, Senechal has written several books and dozens of papers on the subject and is an active member of the International Union of Crystallographers (IUCr). You'd think that if anyone could give you a definition of a crystal, then Senechal could. But she can't.
"We don't know what a crystal is," Senechal confessed to the audience at a session on geometry at last year's meeting of the American Association for the Advancement of Science. In a talk titled "Crystals Aren't What They Used to Be," Senechal explained how the discovery of quasicrystals in the 1980s has led crystallographers to rethink what it means to be a crystal.
As a member of the IUCr Commission on Aperiodic Crystals, Senechal was instrumental in forging a new working definition. But it remains to be seen, she says, what the new view of crystals encompasses or permits.
"The fact is," Senechal says of the title of her AAAS talk, "crystals never were what they 'used to be.' " The scientific study of crystals, she explains, has evolved over the centuries, from simple classifications of gems, through the microscopic inspection of minerals, to investigations of x-ray diffraction patterns. In the early 19th century, the French abb� and mineralogist Ren�-Just Ha�y theorized that crystals consist of tiny "building blocks" arranged according to prescribed rules. Ha�y's theory seemed to account for the full variety of crystals observed in nature. In a crucial experiment carried out a hundred years later, the German physicist Max von Laue tested this theory, along with two other theories of more recent vintage: the existence of atoms and the electromagnetic nature of x-rays.
Von Laue's scientific hat trick "changed modern science," says Senechal. The x-ray diffraction patterns he obtained were a kind of Rosetta stone for crystallography and atomic physics. But they also encouraged a misleading point of view that would prove as tough as diamond. The building-block theory emphasized the most striking spatial property of crystals: the fact that each crystal is a repetitious array of regular parts. This fit well with mathematicians' predilections for symmetry and simplicity. Regularly repeating patterns are subject to a good many geometric and combinatorial restrictions. Mathematicians found that they could step in with explanations and proofs for much of what crystallographers had discovered-all without having to learn any physics or chemistry.
Mathematically, the "reality" underlying a regular array is known as a lattice: the set of all translations that land each member of the array on another member of the array. What distinguishes one crystal from another is the presence or absence of additional symmetries involving rotations and reflections, and the integer-valued normal vectors that define facets. The inexorable conclusion of a lattice-based view of crystals is that crystals are extremely restricted in the rotational symmetries they can have. Only multiples of 60 and 90 degrees are allowed, for a total of four rotational symmetries: 2-fold, 3-fold, 4-fold, and 6-fold. In particular, 5-fold symmetry, with a rotation of 72 degrees, cannot exist in crystals, at least in theory-and therefore should not be found in nature. But it is.
In the early 1980s, Dany Shechtman, a materials scientist at the Technion in Israel and the National Bureau of Standards (now the National Institute of Standards and Technology) in the United States, got some very strange diffraction patterns from an alloy of aluminum and manganese. The pictures very clearly exhibited the forbidden 5-fold symmetry. (To be precise, the symmetry was 10-fold, as if a pair of interdigitating starfish had crept into the field of view.) This couldn't be, Shechtman reasoned, so he repeated the experiment. But the starfish symmetry wouldn't go away. Shechtman then did what any sensible scientist would do: He shelved the result and started asking around.
Finally, a colleague suggested that he read Thomas Kuhn's famous Structure of Scientific Revolutions---the book that shifted the primary context of the word "paradigm" from grammar to science (and on to sociology, where it has lost all meaning whatsoever). Shechtman realized that his reluctance to publish was based more on the tacit, if sensible, acceptance of an existing world view than on the norms of careful scientific experimentation. So he and co-authors Ilan Blech, Denis Gratias, and John W. Cahn published their now-famous paper, "Metallic Phase with Long-range Orientational Order and No Translational Symmetry."
The paper on quasicrystals, as Shechtman's 5-fold symmetric minerals have come to be called, appeared in November 1984 in Physical Review Letters. As luck would have it, an international conference on mathematical crystallography, organized by Senechal and Louis Michel, a mathematical physicist at IHES in Paris, was scheduled for January 1985. Their carefully thought out schedule was completely overshadowed by the quasicrystal discovery, Senechal recalls: "The whole conference turned into a study of that paper."
Mathematically, what immediately took center stage were the geometric shapes known as Penrose tiles. Invented in the 1970s by the mathematical physicist Roger Penrose of Oxford University, Penrose tiles provide a way of tiling the plane in an aperiodic fashion, but with lots of "local" 5-fold symmetry. It would be another triumph of mathematics if quasicrystals turned out to be a manifestation of these simple shapes.
That doesn't seem to be in the cards, though. "Real quasicrystals are not made up of Penrose tiles," Senechal points out. "We don't even know that real quasicrystals are anything like the Penrose tiles." Even so, she adds, Penrose tiles do an amazingly good job of modeling many of the properties of quasicrystals (see Figure 1). "Penrose tiles are a tool for us to try to understand what we mean by long-range order that's not periodic," Senechal says. "They open a door to a wide variety of possible structures."
Which brings us to the new working definition of a crystal. The first job of the IUCr's Commission on Aperiodic Crystals was to define its terms---"and obviously the first term was 'crystal,' " Senechal says. It was clear that the standard, textbook definition, which identifies crystals as having periodic, repeating patterns, wouldn't work. But it was less clear what would work, in large part because crystallographers were (and still are) unsure of the physics that makes quasicrystals possible. As Senechal recalls it, "The question was, How do you define something when you don't know what you're talking about?" Finally, Senechal proposed a working definition that everyone could agree on. A crystal, the commission decided, is any substance that has an x-ray diffraction pattern with sharp, bright spots.
"I felt very strongly we should define it in terms of what we see," says Senechal. "In other words, we recognize these things as crystals because of their diffraction patterns, and so that should be the definition until we know what we're talking about."
The commission's definition "is not as loosey-goosey as it sounds," Senechal says. In purely mathematical terms, an x-ray diffraction pattern is a measure, produced by taking the Fourier transform of the autocorrelation function of a sum of delta functions representing the atoms of the crystal. "Having sharp, bright spots" is a nontechnical way of saying that the measure has a discrete component (which is a fancy way of saying that the measure itself consists, at least in part, of delta functions).
Do crystallographers really need to bone up on Borel sets, inner and outer Lebesgue measure, Schwartz distributions, the Radon-Nikodym theorem, and the other abstruse paraphernalia of measure theory? For the vast majority, the answer is No-no more than architects need concern themselves with the general theory of relativity when designing buildings that won't fall down. Traditional, periodic crystals are still the norm, and they pose challenge enough, Senechal points out. "Most crystallographers are working on hard scientific problems, and worrying about measure theory is really the least of their concerns." But researchers exploring novel materials with "forbidden" symmetries and other affronts to traditional crystallography are clearly going to need some help from mathematicians.
One task now confronting mathematical crystallographers is to figure out what the working definition implies. In particular, what kinds of point sets give rise to measures with a discrete component? (It would be a bit embarrassing if gases turned out to be crystals.) Quite a few researchers, including Robert Moody at the University of Alberta and Jeff Lagarias at AT&T Laboratories-Research are hard at work on the problem, Senechal says. Lagarias, for example, has been studying the relation between local and global properties in point sets known as Delone sets, which are characterized by a lower bound r for the distance between two points in the set and an upper bound R for the distance to the set from a point not in the set.
Questions also arise regarding the "reality" of point sets that do meet the definition. Other than the original 5-fold quasicrystals, for example, researchers have observed only 8-, 10-, and 12-fold symmetries in materials. But mathematically, "we can easily construct tilings and patterns with 7-fold diffraction patterns," Senechal says. "This is one of the puzzles: We've opened up a door now-we can do anything, but nature can't!" That shouldn't be counted as a victory, though. "It's very likely that there's some constraint that we haven't yet grasped," Senechal observes. "It will be very interesting to see what that is."
Barry A. Cipra is a mathematician and writer based in Northfield, Minnesota.

