FDA Researchers Model Virus Transport Through Synthetic Barriers
April 14, 1998
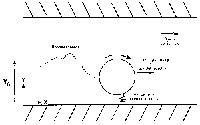
Figure 1. The simplest of the FDA models depicts a pore in a barrier material like latex as a two-dimensional channel and a virus as a sphere. The motion of the virus is affected by convection in the carrier fluid, diffusion, and short-range interactions with the pore wall.
In an old joke about an applied mathematician advising a group of bookies at a horse race, the mathematician begins his presentation, "Consider a spherical horse. . . ."
Scientists at the U.S. Food and Drug Administration have begun at a similar point, considering a spherical virus rather than a horse, but in an environment in which no one wants to gamble. They are modeling the effectiveness of barriers like latex gloves and condoms in stopping the transmission of such deadly viruses as HIV and hepatitis. Their work is no joke: It shows that conventional laboratory tests sometimes seriously underestimate the risk of transmission through such barriers. Customarily, such a product is subjected to a so-called penetration test. The test membrane is placed so that it separates a virus suspension (usually one that is safer to handle than HIV) from a clean collection fluid. Some membranes will transmit viruses because of manufacturing defects or tears; others are naturally porous.
Counting the number of viruses that find their way into the collection fluid over time measures the membrane's effectiveness in stopping virus transmission. Since the FDA regulates the use of barriers of all sorts, as well as the claims that manufacturers can make about their effectiveness, careful quantification through these measurements is essential.
Are Laboratory Tests Accurate?
Matthew R. Myers and his colleagues C. David Lytle, Licia B. Routson, and Bigyani Das at the FDA's Center for Devices and Radiological Health asked a crucial question: Do these static laboratory tests accurately predict the risk of virus transmission in ordinary use with more dangerous viruses? More specifically, what physical properties of the surrogate viruses determine whether they are accurate substitutes for the harmful viruses? How critical is the size of the virus? What is the role of the carrier fluid (e.g., blood, semen, saline)?
To build the understanding required to answer these questions, the FDA team developed a mathematical model of virus transport through the pores of materials like latex. At its simplest, the model depicts the pore as a two-dimensional channel whose width is on the order of ten times the diameter of a virus (see Figure 1). The plane two-dimensional pore geometry duplicates their calibration apparatus, but cylindrical pores were modeled as well for comparison with validation experiments using condoms with laser-drilled pores of known size.
Because most human viruses are approximately spherical, the virus is modeled as a sphere moving through the pore. Its motion is influenced by convection in the carrier fluid, by diffusion, and by short-range interactions with the pore walls. Interactions that trap the virus against the pore wall are the primary barrier to transmission. Modeling the virus as a sphere captures the characteristic that dominates its interaction with the wall, i.e., the size of the virus relative to the size of the pore.
Although the interactions between the virus and the pore wall are ultimately governed by complex intermolecular forces, the FDA team cleverly subsumed the difficulties of modeling those forces into an expression involving a single parameter, one that is relatively easy to determine in laboratory experiments. Once calibrated, their model can predict the risk of virus transport in settings not usually considered by conventional barrier tests.
Traveling Through the Pore
Convection in the carrier fluid dominates diffusion in carrying the virus through a pore in the barrier, but diffusion is the primary mode of transport across the pore. If a virus moves sufficiently slowly through the pore, diffusion may bring it close enough to the wall for molecular forces to hold it there. In effect, the wall can function as a sink for the virus.
Standard models for convection and diffusion apply in this setting. In addition, a number of well-regarded expressions describing the intermolecular forces appear in the literature. Unfortunately, even some of the simplest involve a total of three empirical parameters, none of which is easily measured in the laboratory.
One component of the intermolecular forces is a van der Waals term arising from the distribution of electric dipoles across the virus and membrane molecules. For latex and the viruses of interest to the FDA, these forces are typically attractive. If the pore wall is a plane, they depend on virus diameter, distance to the wall, and a single empirical parameter. The second component of the intermolecular forces arises from the so-called electric double-layer force induced by the net difference in electrical charge between the membrane wall and the virus. Typically, a virus and the membrane are both negatively charged, so that this force is repulsive. A careful mathematical model of this force is out of the question because the source term in the electric potential equation is nonlinear. A widely accepted approximation still requires two more parameters, the surface potentials of the virus and the membrane.
One Parameter Stands in for Three
Myers and his colleagues circumvented the three-parameter problem of the "van der Waals model" by dividing the pore into two regions in which different mechanisms determine virus transport. One of the regions is a thin boundary layer along the pore wall. Here convection is ignored; diffusion and the short-range intermolecular forces between the virus and the wall dominate. Within the boundary layer, the balance between wall forces and diffusion leads to an expression for virus concentration as a function of distance from the wall.
In the balance of the channel, outside the boundary layer, the virus is free of interaction forces. The virus is carried along by the velocity profile computed for the carrier fluid; that is, convection is the dominant mode of virus transport in the region away from the pore wall.
At the boundary between the two regions, which is located a short distance (the length of a few virus diameters) from the pore wall, the model requires that virus concentration and virus flux normal to the wall balance, as if an actual permeable barrier separated the boundary layer from the main part of the channel. The single permeability parameter for that imaginary barrier, a sort of rate constant, replaces the three empirical constants required to model the van der Waals and electric double-layer forces. (The matching between the two regions occurs at a fixed distance from the pore wall, not through matched asymptotic expansions.)
To calibrate the model, Myers's team constructs a long, narrow, two-dimensional channel by separating two sheets of latex condom material with thin spacers (much as two bookmarks placed side by side on the same page would create a narrow channel through a closed book; see Figure 2). The researchers measure the fraction of virus transmitted down the channel over various time intervals, then use the secant method to compute the particular value of the rate constant at the boundary layer that best predicts the measured transmission values. Finite differences and the method of lines are used to solve the convection-diffusion equations outside the boundary layer.
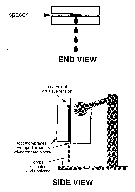
Figure 2. Calibration of the model: The fraction of virus transmitted through the channel is measured at various time intervals, and the value of the rate constant at the boundary layer that best predicts the measured transmission values is calculated.
Calibration with their single parameter is obviously much easier than the three-parameter fit required by the van der Waals model. It turns out that computing the three parameters would be even harder than it first appears--the intermolecular force expressions are singular near the boundary, requiring an especially dense computational grid.
Troubling Predictions
Myers and his colleagues set out to identify "situations where the risk associated with membrane use is underestimated by a given [laboratory] test." Their model suggests some troubling possibilities.
Using bacterial viruses over a range of pore sizes, the model predicts that the transmission rates for smaller pores are highly dependent on the type of virus (or, more precisely, on the rate constant for the particular virus and membrane). Under identical conditions, for example, the predicted probabilities of transmission through a pore 0.5 micron in diameter are about 97% for one virus and less than 2% for a different virus, even though the diameter of each virus is less than 20% of the pore diameter. A conclusion about barrier integrity based on tests with just one virus could therefore be dangerously misleading.
A second concern is the variation in transmission rates with the ionic concentration of the carrier fluid. Such variations occur naturally with fluctuations in sodium or potassium levels in blood and with cyclic pH variations in vaginal fluid. The model shows that small changes in ionic concentration can lead to changes of orders of magnitude in relative transmission rates. At higher ionic concentrations, for example, it appears that repulsive electrical forces are blocked by the electric field of the ions in the fluid. Consequently, testing a membrane at ionic concentrations higher than those occurring in practice or testing with a virus with a weaker charge can result in significant underestimation of the risk associated with use of the membrane.
The same phenomenon occurs with filters used in biomedical devices or in surgical masks and gowns. These filters serve as barriers to the passage of viruses suspended in carrier fluids that can vary widely in ionic concentration. In these settings, the salinity of the suspending fluid, for example, can strongly influence the effectiveness of the filter.
Future Work
This recent work has already shown its value in explaining once-puzzling FDA test results, such as why the addition of small amounts of certain liquids (e.g., serum diluted to 0.1%) to a carrier fluid would change virus transmission from almost 0 to nearly 100%. But the full impact of the model will be seen when it is made available to manufacturers of gloves and condoms, provoking additional careful review of its implications. The FDA team hopes that the model will also help developers of new barrier materials simulate the performance of their products under a wide range of conditions, including nonstatic uses.
Myers suggests that the reception their work receives will depend in part on "the parameter ranges for which we can calibrate our model. HIV is hard to study in the laboratory because it is so dangerous. Furthermore, laboratory penetration tests are usually performed in saline solution, even though blood is the natural environment for HIV, because of the practical difficulties associated with acquiring and handling human blood.
"More generally, anything that's alive in the laboratory can become contaminated or die, leading to low quantitative accuracy and difficulty in calibrating any model. Indeed, about half of our time on this project--and most of our money--goes into experiments!"
Much of the team's reported validation work concentrated on latex barriers, because the laser-drilled pores in those barriers closely approximate the cylindrical geometry assumed in the model. Other geometries, such as channels with elliptical cross sections that model tears in gloves, are now receiving more attention. Winding-tube and porous-media models, which are particularly appropriate for gowns and masks, are also being developed.
Meanwhile, mathematical modeling is in the difficult position of pointing to increased risk in an already risky environment.
Paul Davis is a professor in the Department of Mathematical Sciences at Worcester Polytechnic Institute.

